Great Info About Does A Positive Electron Exist
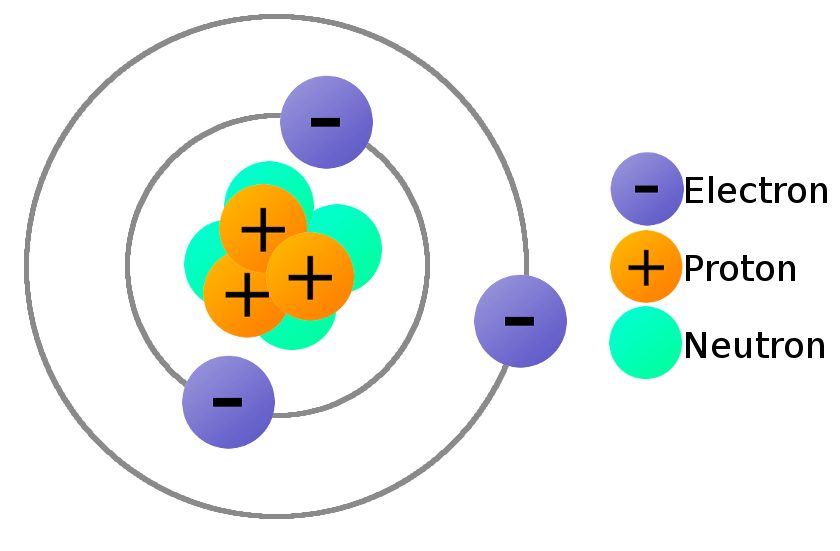
Electron Proton Khám Phá Sâu Về Hai Hạt Cơ Bản Trong Vật Lý Nhân
The Curious Case of the Positive Electron
1. Unveiling the Positron
So, you're wondering if a positive electron exists? Well, hold onto your hats, because the answer is a resounding yes! But, technically, we don't call it a positive electron. Instead, physicists use a much cooler name: the positron. Think of it as the electron's mischievous twin from another dimension (okay, maybe not another dimension, but close!).
The positron is the antiparticle of the electron. What does that mean? It means it has the same mass as an electron but carries a positive electric charge instead of a negative one. It's like looking in a mirror — the image is the same, but flipped. Instead of being repelled by other negatively charged particles, positrons are attracted to them. Opposites really do attract, even in the subatomic world.
The existence of the positron was predicted way back in 1928 by a brilliant physicist named Paul Dirac. He was tinkering with his equations trying to combine quantum mechanics and special relativity, and his calculations suggested the possibility of a particle identical to the electron but with the opposite charge. Now that's some serious brainpower!
It took a few years, but in 1932, Carl Anderson, while studying cosmic rays, actually observed a positron in a cloud chamber. The cloud chamber is a device that allows you to see the paths of charged particles. He saw a track that looked exactly like an electron, but it was bending in the opposite direction in a magnetic field. Boom! Confirmation of the positron. Anderson won the Nobel Prize for this discovery, and rightfully so.

Hunting Down the Positron
2. Positron Sources
Okay, so we know positrons exist, but where do we find these elusive particles? They're not exactly hanging out in your living room (unless you have a very sophisticated particle accelerator hidden behind the sofa). Positrons are created in a few different ways, often from high-energy processes. One common source is radioactive decay. Certain radioactive isotopes, when they break down, can emit positrons.
Another place where positrons are found is in the aftermath of high-energy particle collisions. When particles smash into each other at incredibly high speeds (like in particle accelerators), energy is converted into matter, and sometimes that matter includes positrons. These collisions mimic processes that happen in the hearts of stars and during supernova explosions. Think of it as a high-speed demolition derby, but with subatomic particles.
Believe it or not, even bananas can emit positrons! That's because they contain a small amount of the radioactive isotope potassium-40. While the amount is tiny and completely harmless, it's a fun fact to impress your friends with at parties (or maybe just to keep in your back pocket for a particularly boring conversation).
Positron Emission Tomography (PET) scans also make use of positrons. In PET scans, a radioactive tracer that emits positrons is injected into the body. When a positron encounters an electron in the body, they annihilate each other, producing gamma rays that can be detected by the scanner. This allows doctors to create detailed images of organs and tissues.
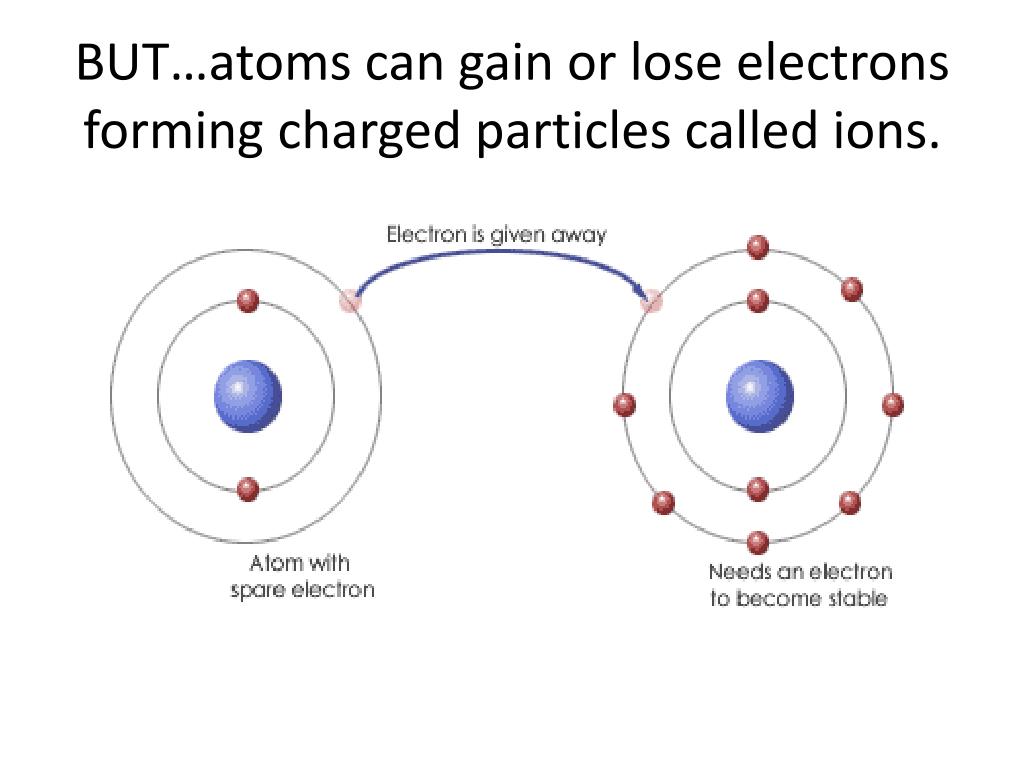
Positively Charged Ion Oilmilo
Annihilation Antics
3. The Explosive Reunion
Now for the really mind-blowing part: when a positron meets an electron, they don't just politely say hello and go their separate ways. Oh no, they annihilate each other! That's right, they completely disappear, converting their mass into energy in the form of photons (light). It's like a tiny explosion of pure energy, a direct demonstration of Einstein's famous equation, E=mc. Think of it as the ultimate recycling program: matter gets turned back into energy.
This annihilation process is what makes positrons so short-lived. As soon as they encounter an electron, poof! They're gone. This is why they don't just hang around like regular electrons — they're always on the lookout for a partner to annihilate with. It's a bit of a dramatic existence, if you think about it.
The photons produced in electron-positron annihilation have a very specific energy, which allows scientists to easily detect them and study the process. This phenomenon is not just a theoretical curiosity; it has real-world applications, as we saw with PET scans earlier.
The annihilation of matter and antimatter is a fundamental process in the universe. It may have played a crucial role in the early universe, helping to shape the cosmos as we know it. Understanding this process is key to unlocking some of the biggest mysteries about the origin and evolution of the universe.
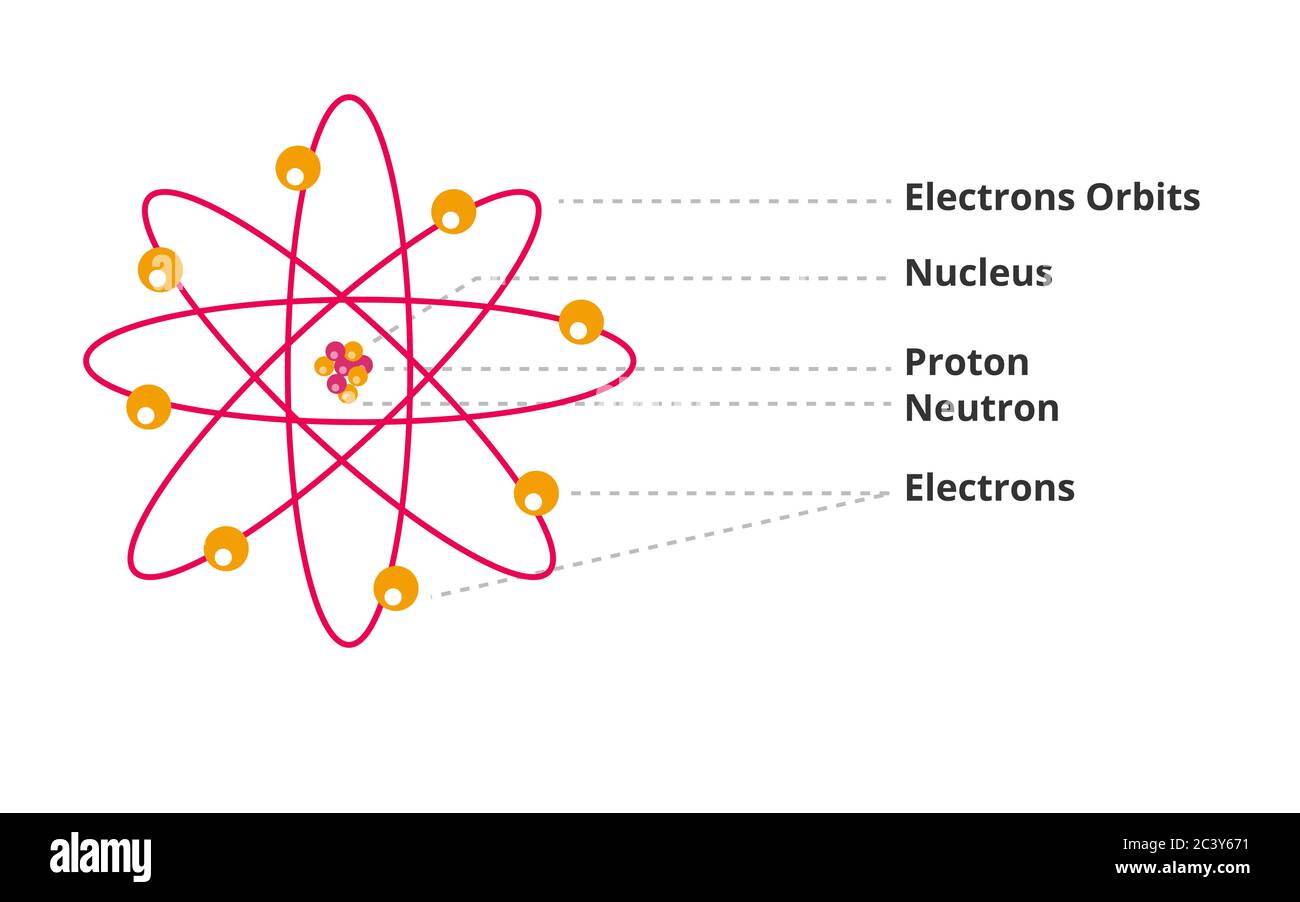
Positrons in Action
4. From Medical Imaging to Materials Science
While the concept of a "positive electron" (positron) might seem purely theoretical, it has some pretty cool real-world applications. One of the most important is Positron Emission Tomography (PET) scans, which we touched on earlier. These scans are used to diagnose a wide range of medical conditions, including cancer, heart disease, and neurological disorders. By tracking the annihilation of positrons in the body, doctors can get a detailed picture of what's happening inside, often detecting problems before they become serious.
Beyond medicine, positrons are also used in materials science. A technique called Positron Annihilation Spectroscopy (PAS) uses positrons to probe the properties of materials. By studying how positrons interact with a material, scientists can learn about its structure, defects, and composition. This can be helpful for developing new and improved materials for everything from electronics to aerospace applications.
Scientists are even exploring the possibility of using positrons in future technologies, such as fusion reactors. Fusion, the process that powers the sun, requires extremely high temperatures and pressures. Positrons might be used to help confine and control the plasma (superheated gas) in fusion reactors, potentially leading to a clean and sustainable energy source. It's a long shot, but the potential payoff is huge.
The study of positrons continues to push the boundaries of our understanding of the universe. As we learn more about these fascinating particles, we may uncover even more unexpected applications and gain new insights into the fundamental laws of nature.

Frequently Asked Questions (FAQ) About Positive Electrons (Positrons)
5. Your Positron Queries Answered!
Still scratching your head about positive electrons (positrons)? No problem! Here are some frequently asked questions to clear things up.
Q: What's the difference between an electron and a positron?A: The positron is the antiparticle of the electron. They have the same mass, but the electron has a negative charge, and the positron has a positive charge. Think of them as mirror images of each other in terms of charge.
Q: Why don't we see positrons everywhere?A: Positrons are very short-lived because they quickly annihilate with electrons, turning into energy (photons). Since there's lots of matter (and therefore lots of electrons) around, positrons don't stick around for long.
Q: Can I make a positron at home?A: Probably not, unless you have access to a particle accelerator or a radioactive source! Positrons are typically created in high-energy processes that require specialized equipment. Stick to baking cookies for now — it's much safer!
Q: Are positrons dangerous?A: In everyday situations, positrons pose no threat. PET scans, which use positrons, are a safe and common medical procedure. The amount of radiation exposure is carefully controlled. However, handling high concentrations of antimatter could be dangerous, but that's not something you'll encounter in your daily life.
