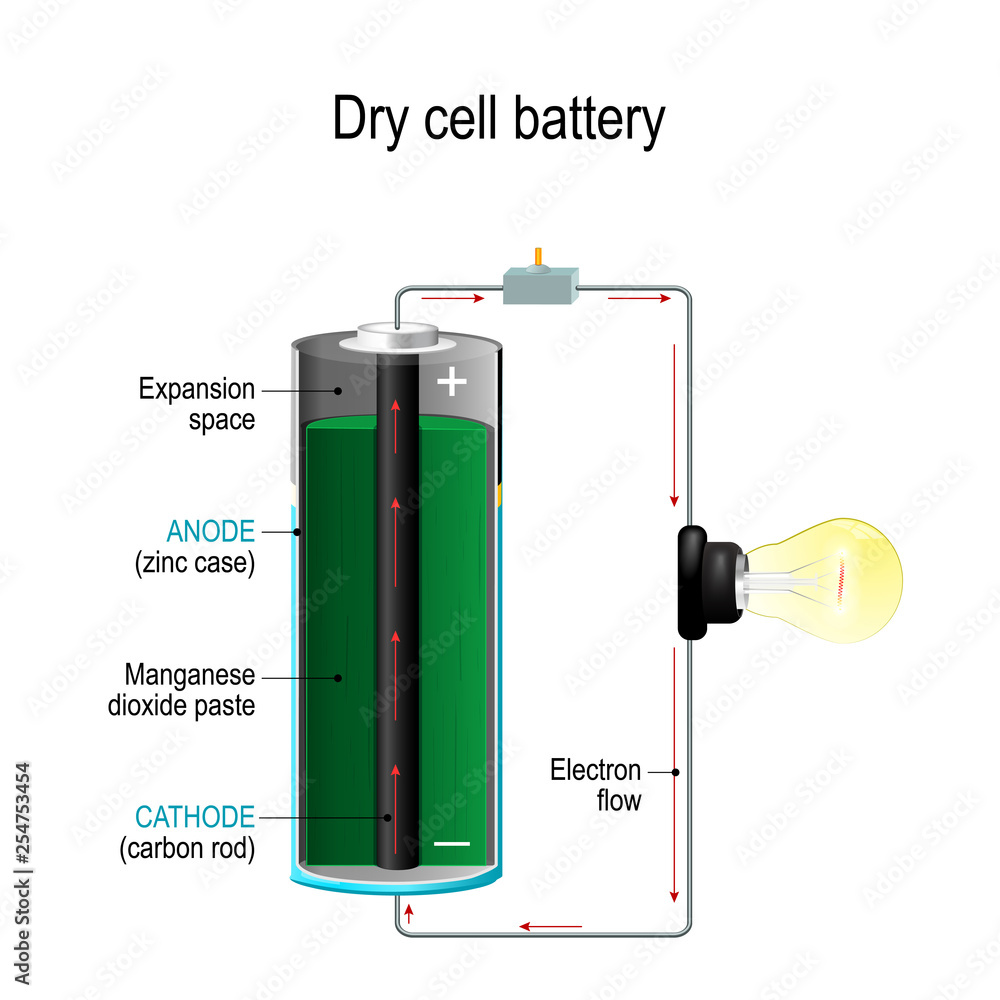
First Class Tips About Do Electrons Flow Out Of A Battery

Premium Vector Electron Flow And Current Electric
The Curious Case of Battery Electrons
1. Unpacking the Mystery
Alright, let's dive into a question that's probably sparked (pun intended!) in your mind at some point: Do electrons actually flow out of a battery? It's a deceptively simple question with a slightly complex answer. The short version is, yes, electrons do move, but its not quite like water flowing from a tap. Think of it more like a meticulously choreographed dance within the electrical circuit.
Imagine a long line of people holding hands. When you push one person at the end of the line, the movement isnt instant, but a wave travels down the line, causing the person at the other end to move almost immediately. That's similar to what happens with electrons in a wire connected to a battery. The electrons themselves dont necessarily zoom from the battery all the way through your device. Instead, they nudge their neighbors, creating an electrical current.
The battery's role is to provide the potential for this movement — the electrical "push." It's like a water pump creating pressure in a pipe. The electrons already exist in the wires of your circuit, waiting for that push to start moving. The battery provides the energy that gets them going and keeps them going.
So, while electrons are indeed flowing within the circuit, including the battery itself, it's more accurate to say the battery facilitates the movement of electrons rather than acting like a reservoir that's constantly emptying. Its a subtle but important distinction. Think of it like this: a battery doesnt give electrons, it motivates them.

Electrolytic Cells Definition, Parts & Uses Lesson
From Chemical Reactions to Electron Motion
2. The Inner Workings
Now, let's peek inside the battery to understand where this "motivating force" comes from. Batteries are basically tiny chemical reactors. They contain different materials that react with each other, and this reaction is what causes electrons to be liberated and set in motion. These reactions don't create electrons, they just shuffle them around, making it easier for them to move within the circuit.
In a typical battery, you have two electrodes (the positive and negative terminals) made of different materials and an electrolyte solution. The chemical reactions at each electrode result in one electrode having an excess of electrons (the negative terminal) and the other having a deficiency of electrons (the positive terminal). This creates a voltage difference — an electrical pressure — that's ready to drive electrons through an external circuit when connected.
When you connect a wire between the terminals, the electrons at the negative terminal are attracted to the positive terminal. They start to flow through the wire, doing work along the way (powering your phone, lighting a bulb, etc.). The battery continues to drive this flow until the chemical reactions inside reach equilibrium, and the battery is considered "dead." Its not that the battery is empty of electrons, but rather that it can no longer maintain the voltage difference needed to push them along.
The cool thing is, in rechargeable batteries, you can reverse the chemical reactions by applying an external voltage. This restores the original conditions and allows the battery to be used again. Its like rewinding a spring to store energy.

The "Electron Flow" vs. "Conventional Current" Conundrum
3. A Historical Mix-Up
Here's a fun fact that often trips people up: In electrical engineering, we often talk about "conventional current" flowing from positive to negative, which is the opposite direction of actual electron flow. Why? Because back in the day, before we knew about electrons, scientists assumed that positive charges were the ones moving.
Benjamin Franklin, the kite-flying, electricity-experimenting legend, arbitrarily assigned positive and negative charges. Turns out, he guessed wrong about which charge carrier was actually moving. But by then, the convention was already established, and changing it would have been a monumental task. So, we're stuck with a system where the "current" is said to flow in the opposite direction of electron movement.
It's kind of like driving on the left side of the road in some countries — it's different, but it works as long as everyone follows the same rules. So, don't be alarmed if you see diagrams or explanations that show current flowing from positive to negative. Just remember that the electrons themselves are actually heading the other way.
Ultimately, whether you think of it as electrons flowing from negative to positive or conventional current flowing from positive to negative, the end result is the same: electrical energy powering your devices. It's all about understanding the underlying principle of charge movement.

Electron Flow In Circuit Diagram Physcis
Beyond the Battery
4. The Closed Loop
Lets remember one crucial point: electrons need a closed loop to flow. A battery sitting on a table, even if its brimming with potential, won't do anything on its own. It needs to be connected to a circuit — a complete path for the electrons to travel from the negative terminal, through a device (like a light bulb or a motor), and back to the positive terminal.
Think of it like a water park slide. You need a continuous slide for the water (and you!) to flow from top to bottom. If there's a break in the slide, the flow stops. Similarly, if there's a break in the electrical circuit (like a broken wire or an open switch), the electron flow stops, and your device won't work.
Switches are a perfect example of this. When a switch is "on," it closes the circuit, providing a continuous path for electrons to flow. When the switch is "off," it opens the circuit, creating a gap that stops the electron flow. This simple concept is the foundation of all electronic devices.
So, the next time you flip a switch, remember that you're not just turning something "on" or "off." You're actually completing or breaking a circuit, allowing or preventing the flow of electrons and bringing your electronic gizmos to life!
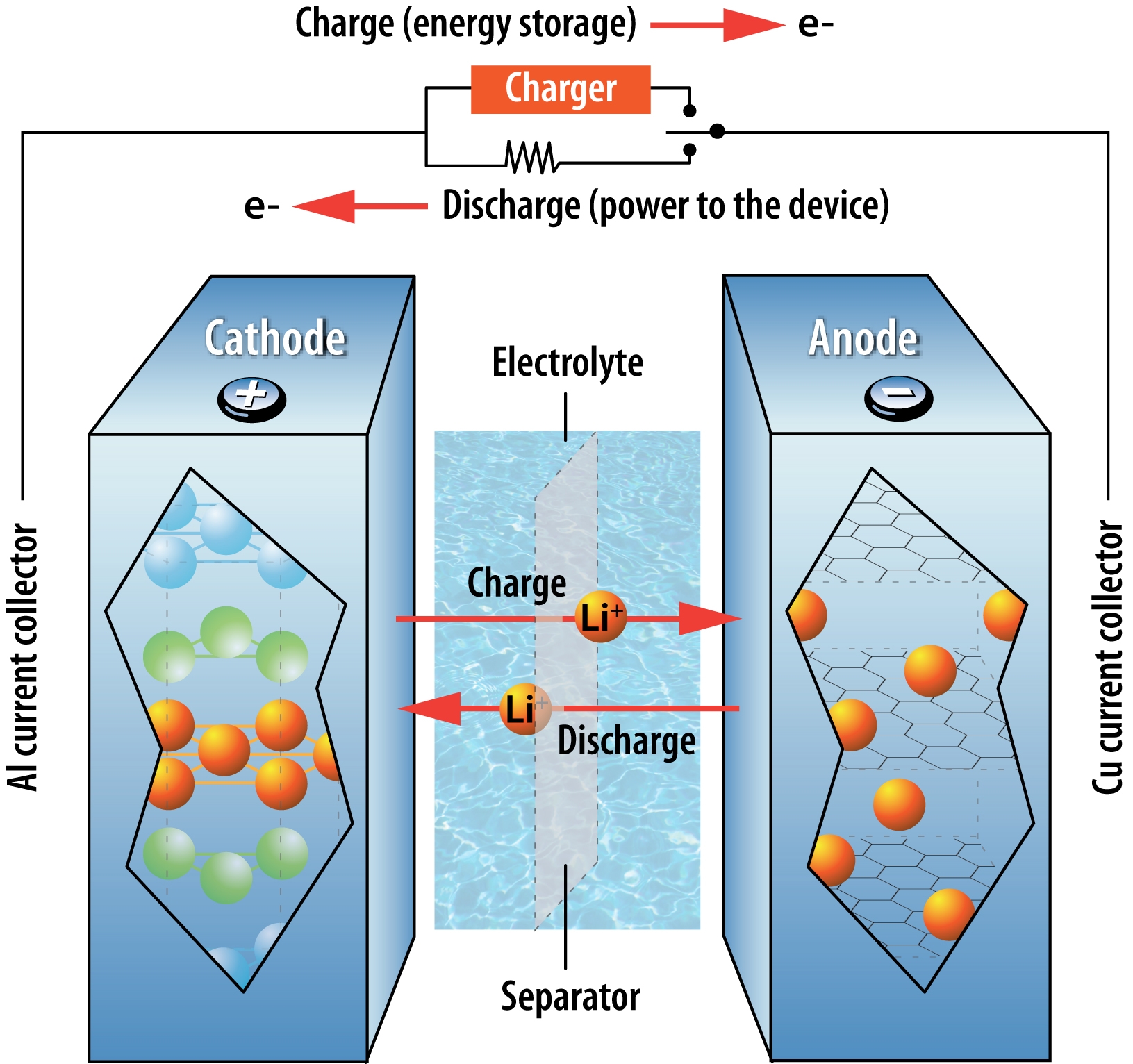
Five Emerging Battery Technologies For Electric Vehicles
FAQs
5. Frequently Asked Questions About Electron Flow in Batteries
Let's tackle some common questions about electrons and batteries:
Q: Do batteries "run out" of electrons?
A: Not exactly. They run out of the ability to move the electrons effectively. The chemical reactions that provide the electrical push stop working, so the battery can't maintain a voltage difference. The electrons are still there, they just aren't motivated anymore.
Q: Are electrons used up in a circuit?
A: Nope! Electrons aren't consumed or destroyed when they power a device. They simply transfer energy. Think of them like tiny messengers carrying energy from the battery to the device and then returning to the battery to get more. The same electrons keep flowing around and around.
Q: What happens to the electrons when a battery is completely discharged?
A: The electrons are still present within the battery materials. The chemical reactions have reached a state of equilibrium, where there's no longer a significant voltage difference between the electrodes. In rechargeable batteries, these reactions can be reversed by applying an external voltage, which essentially "recharges" the electron-moving mechanism.
Q: Is electron flow the same in all types of batteries?
A: The basic principle is the same (chemical reactions driving electron movement), but the specific reactions and materials used can vary greatly between different types of batteries (e.g., alkaline, lithium-ion, lead-acid). This affects things like voltage, energy density, and lifespan.